HIV and COVID-19 vaccines On December 16, 2020, the online journal The Poz reported on the COVID-19 vaccine developed by Pfizer and BioNTech. The world’s first COVID-19 vaccine approved for actual use (beyond clinical trials) in various countries, including the United States. The FDA approved the vaccine for emergency use authorization (EUA).
In the article written by Liz Highleyman, a key question was posed: Is this vaccine safe for people living with HIV ? This is particularly relevant as individuals with HIV may be considered immunocompromised. Or might be on medications that affect their immune system. The article summarized key points as follows:
The Pfizer-BioNTech COVID-19 vaccine was the first COVID-19 vaccine approved for use in the United States. The FDA granted emergency use authorization (EUA) on December 11, 2020, following strong support from the vaccine advisory committee. Which concluded that the benefits outweighed potential risks.

COVID-19 Vaccine Approval and Recommendations for People Living with HIV
The Pfizer-BioNTech COVID-19 vaccine became the first COVID-19 vaccine approved for emergency use in the United States on December 11, 2020. This approval followed strong support from the vaccine advisory committee, which concluded that the benefits outweighed any potential risks. Following this approval, a second vaccine, developed by Moderna in collaboration with the NIH. Using similar mRNA technology, was also expected to receive approval soon.
As the vaccines began to be distributed across the U.S. People living with HIV raised concerns about the suitability of the vaccines. For their condition and where they would fall in the vaccination priority schedule. Current evidence indicates that COVID-19 vaccines are safe for people with HIV. And advocacy groups have called for prioritizing individuals with HIV in early vaccination phases.
The development of COVID-19 vaccines occurred at an unprecedented speed. With Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases. Initially predicting a 12 to 18-month timeline for vaccine development. Despite the rapid approval, concerns and misinformation about vaccine safety spread, including claims that people with HIV should delay vaccination.
However, most experts, including Dr. Monica Gandhi, medical director of the Ward 86 HIV Clinic at Zuckerberg San Francisco General Hospital. Strongly recommend COVID-19 vaccination for people living with HIV.
Safety and efficacy of vaccines
Inclusion of People with HIV in COVID-19 Vaccine Trials
Both Moderna and Pfizer/BioNTech adjusted their inclusion criteria to allow people with HIV to participate in their COVID-19 vaccine trials. Following successful advocacy campaigns. The Pfizer/BioNTech trial included 120 participants with HIV. Though they were excluded from the primary efficacy and safety analyses due to being in a later-stage trial. Moderna’s trial, on the other hand, included 176 participants with HIV.
In both trials, a participant with HIV who received the placebo (mock vaccine) became infected with the SARS-CoV-2 virus and developed symptomatic COVID-19. However, no individuals with HIV who received the experimental vaccine developed symptomatic COVID-19. And there were no reports of unusual safety concerns.
Other ongoing COVID-19 vaccine trials, including those developed by AstraZeneca, Johnson & Johnson, Novavax, and Sanofi/GlaxoSmithKline, are also including people with HIV as part of their participant pools.
Considerations for HIV-infected people and COVID-19 vaccines
Concerns About Adenovirus-Based Vaccines and HIV Risk
In October, Professor Susan Buchbinder from the University of California, San Francisco, and her colleagues raised concerns in The Lancet about the use of adenoviruses, particularly adenovirus type 5 (Ad5), in COVID-19 vaccines. These vaccines use the common cold virus to deliver the SARS-CoV-2 protein genes into the body.
The concerns stem from past research, particularly the STEP (Phambili) HIV vaccine project, which used an HIV vaccine with an Ad5 vector. This study found that the vaccine increased the risk of HIV infection in men who have sex with men and already had immunity to adenovirus type 5 from previous exposure. Researchers suggested that pre-existing immunity to Ad5 could facilitate the proliferation of HIV in CD4 T cells or make those cells more susceptible to HIV infection.
The authors of the study warn that the use of Ad5 as a vector in SARS-CoV-2 vaccines may increase the risk of HIV infection, particularly in vaccinated men. This issue of vaccine safety needs to be carefully considered before further development of Ad5-based COVID-19 vaccines.
Vaccine Safety and Efficacy for People with HIV
Vaccines such as Pfizer/BioNTech and Moderna, which do not use adenoviruses, are generally considered safe for individuals living with HIV. AstraZeneca/Oxford vaccines, which utilize chimpanzee adenoviruses, also pose no significant risk regarding human immunity. However, vaccines like the CanSino Biologics vaccine, which use human adenovirus type 5 as a vector, may carry a potential risk for those with HIV due to pre-existing immunity to the virus.
Despite these concerns, both the British HIV Association (BHIVA) and the Terrence Higgins Trust have affirmed that the Pfizer/BioNTech and AstraZeneca/Oxford vaccines are safe for people living with HIV. Neither vaccine contains live virus, reducing the risk for individuals with compromised immune systems. Nonetheless, these organizations also note that people with HIV may experience a weaker immune response to the vaccine, potentially making the vaccines less effective for this group.
Should people with HIV be prioritized [for vaccination]?
Prioritizing People with HIV for COVID-19 Vaccination
Stage two of the COVID-19 vaccination rollout includes individuals with pre-existing conditions. That moderately increase their risk, such as asthma, hypertension, and immunosuppression caused by factors like a bone marrow transplant, HIV/AIDS, liver disease, corticosteroid use, or pregnancy. In the UK, people with HIV are categorized into group 6 (out of 9), which covers individuals up to 64 years old with conditions elevating their risk of severe illness or death. Those over 65, regardless of HIV status, are placed in higher-priority groups.
The British HIV Society highlights that some individuals with HIV. May meet the criteria for group 4 (clinically very high risk). These include those with a CD4 count below 50, individuals with a history of severe immunosuppression. A current CD4 level below 200 coupled with other risk factors such as a detectable viral load or additional comorbidities. And those who have recently experienced severe HIV complications.
Although smaller studies have not identified a direct link between HIV and elevated risks of SARS-CoV-2 infection or severe COVID-19, larger studies have begun to show an association. This emerging evidence, alongside an understanding of overlapping risk factors among people living with HIV, has prompted action.
A coalition of advocacy groups—including the Black AIDS Institute, Let’s Kick ASS (AIDS Survivor Syndrome), NASTAD, Prevention Access Campaign, SisterLove, Treatment Action Group, and the Well Project—has issued an open letter urging the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) to prioritize people living with HIV in the COVID-19
Experiences of COVID-19 vaccine research participants
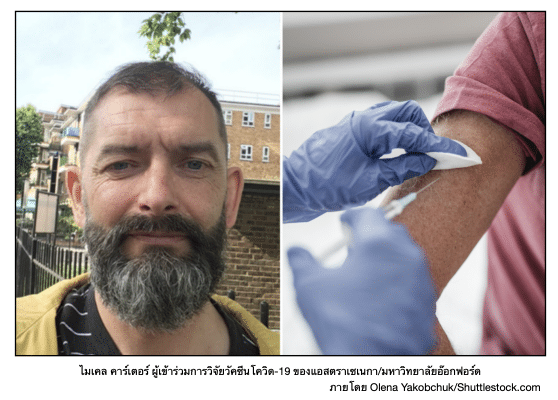
Michael Carter: A Testament to Science Saving Lives
Michael Carter of the UK reflected on the profound impact of scientific advancements. Comparing the development of an effective HIV treatment—one that saved him from premature death 30 years ago—to the strides made in creating a COVID-19 vaccine. Now in his fifties, Carter credits these achievements with preserving his health and life. Recently, he participated in the AstraZeneca/Oxford University COVID-19 vaccine trial, a sub-study specifically designed to evaluate the vaccine’s safety and effectiveness in people living with HIV. Unlike other vaccine trials, this study ensured that all participants received the experimental vaccine, omitting the use of mock vaccines.
Joining the Vaccine Sub-Study
Carter joined the sub-study after the main phase 3 clinical trial, which included over 20,000 participants across Brazil, South Africa, and the UK. The primary trial aimed to compare side effects between those receiving a placebo and those receiving the experimental vaccine. Before participating, Carter underwent a rigorous medical screening to confirm eligibility, including a physical exam and a detailed review of his medical history.
Detailed Insights and Safety Measures
Researchers provided comprehensive information about the vaccine’s mechanism—using inactivated SARS-CoV-2 virus to stimulate immune responses—and its most common side effects, such as mild soreness and flu-like symptoms lasting up to two days. They also explained a temporary halt in the trial caused by a rare neurological issue in another participant, later deemed unrelated to the vaccine.
Carter’s health was closely monitored throughout the study. He underwent regular blood tests and reported any side effects via an online diary. Researchers ensured transparency, sharing study results promptly and inviting participants to ask questions.
Carter’s Perspective on Vaccine Efficacy
While acknowledging that the AstraZeneca/Oxford vaccine’s efficacy was lower than two previously announced vaccines, Carter remained optimistic. He was pleased that none of the vaccinated participants required hospitalization due to COVID-19 and emphasized the importance of having multiple vaccines to combat the pandemic effectively.
Personal Motivation and Gratitude
Carter’s decision to join the study was deeply personal, as his father had succumbed to COVID-19 shortly before his participation. He expressed immense gratitude to the scientists, medical professionals, and fellow volunteers for their dedication to developing a safe and effective vaccine.
Global Vaccine Studies and Inclusion of People with HIV
Carter’s trial is one of many—including studies funded by the US government’s Operation Warp Speed—that have started enrolling people living with HIV. These efforts aim to provide sufficient data on vaccine safety and efficacy for this vulnerable group, though outcomes will depend on the number of HIV-positive participants in each study.
Thailand’s Vaccine Strategy and HIV Concerns
In November, the Thai government secured 26 million doses of the AstraZeneca/Oxford vaccine, sufficient for 13 million people. Additionally, a deal was signed to produce the vaccine domestically. However, limited initial supplies necessitate prioritizing high-risk groups, raising questions about whether people with HIV will be included. Advocates stress the importance of amplifying the voices of individuals with HIV to ensure their risks and vaccine needs are addressed in Thailand’s national plan.
References and thanks for the content
Last Updated on 18/12/2025 by ทีมที่ปรึกษา มูลนิธิเพื่อรัก