The global HIV epidemic remains a major public health challenge, particularly among high-risk populations such as men who have sex with men (MSM), individuals with HIV-positive partners, and people who inject drugs (PWID). To strengthen HIV prevention efforts, Gilead Sciences has developed a new medication called Lenacapavir, which has demonstrated up to 96% effectiveness in reducing HIV infections with dosing required only twice per year. This breakthrough positions Lenacapavir as a highly promising next-Gen PrEP option for HIV prevention.

Overview of Next-Gen PrEP Option
Lenacapavir is a multi-stage HIV inhibitor, distinguishing it from traditional antiretroviral drugs that typically act on a single stage of the viral replication cycle. By targeting multiple phases of the HIV life cycle, Lenacapavir has the potential to provide more robust and sustained protection against infection. In addition to its preventive potential, Lenacapavir has already been approved in several countries for the treatment of adults living with multi-drug-resistant HIV, further underscoring its clinical significance.

Clinical Trials and Outcomes of Lenacapavir
PURPOSE 2 is a global Phase 3, randomized, double-blind, multicenter clinical trial designed to evaluate the safety and efficacy of subcutaneous Lenacapavir injections administered twice yearly as PrEP, compared with daily oral Truvada and baseline HIV incidence (bHIV).
The study enrolled more than 3,200 participants aged 16 years and older, including cisgender men, transgender men, transgender women, and non-binary individuals who have sex with partners assigned male at birth. Trial sites were located across Argentina, Brazil, Mexico, Peru, South Africa, Thailand, and the United States, spanning 88 clinical centers.

Participants were randomized in a 2:1 ratio, with one group receiving Lenacapavir and the other receiving daily Truvada. Given the existence of proven PrEP options, the use of a placebo was considered unethical. As a result, baseline HIV incidence served as the primary comparator, while Truvada functioned as a secondary comparator.
Among the 2,180 participants receiving Lenacapavir, only two HIV infections were reported, corresponding to an incidence rate of 0.10 per 100 person-years. This means that 99.9% of participants in the Lenacapavir group remained HIV-negative. In comparison, baseline HIV incidence was 2.37 per 100 person-years, translating to a 96% reduction in infection risk (hazard ratio 0.04, p < 0.0001).

In the Truvada group, nine infections occurred among 1,087 participants, with an incidence rate of 0.93 per 100 person-years. Overall, twice-yearly Lenacapavir demonstrated 89% greater effectiveness than daily Truvada (hazard ratio 0.11, p = 0.00245). Both medications were well tolerated, with no new major safety concerns identified.
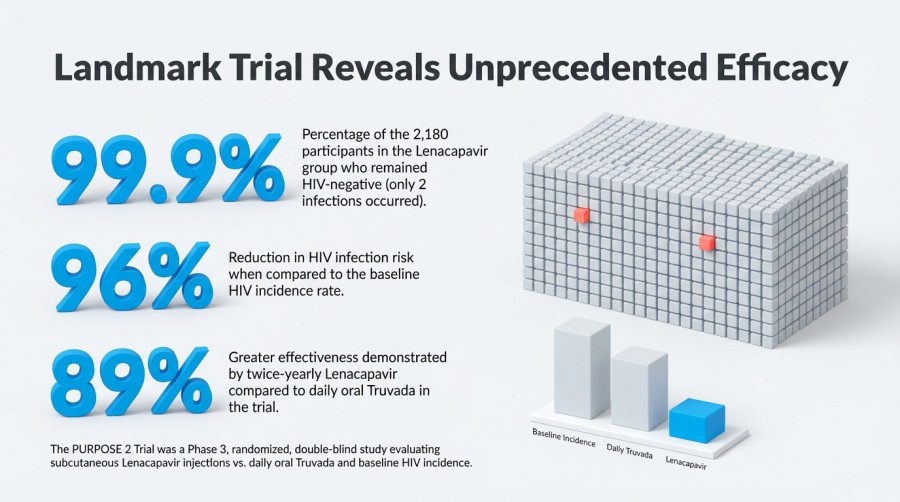
Summary of the PURPOSE 2 Trial
The Phase 3 PURPOSE 2 trial (NCT04925752) represents a pivotal milestone in evaluating Lenacapavir as a Next-Gen PrEP Option. Interim analysis revealed remarkable outcomes:
- 96% reduction in HIV acquisition risk compared with baseline incidence
- Extremely low infection rate, with only 2 infections among 2,180 participants
- Superior efficacy to daily Truvada, achieving 89% greater protection
- Strong safety profile, with good tolerability and no significant new safety signals
These findings reinforce the potential of Lenacapavir to transform HIV prevention strategies.
The Importance of Transforming HIV Prevention Models
The ability to administer Lenacapavir just twice per year is especially significant, as many individuals struggle with daily pill adherence. The PURPOSE 2 trial results offer renewed optimism for developing prevention methods that are not only effective but also simpler, more accessible, and less stigmatizing for people at high risk.
“The difficulty many people face in taking a daily pill—along with adherence challenges and stigma—has long hindered access to and persistence with standard prevention options. These impressive results give us new hope in reducing HIV acquisition.”
— Onyema Ogbuagu, Principal Investigator, PURPOSE 2 Trial
Expanding Global Access to Lenacapavir
Gilead Sciences has outlined a global access strategy aimed at ensuring Lenacapavir reaches those who need it most, particularly in high-burden, resource-limited settings. The company plans to initiate global regulatory submissions by the end of 2024, potentially supporting market availability in 2025.
“We will work closely with regulators, governments, and public health partners to ensure that, if approved, Lenacapavir can be delivered to people who need it.”
— Daniel O’Day, Chairman and CEO, Gilead Sciences
Supporting Evidence from Additional Clinical Trials
Data from the PURPOSE 1 trial, which evaluated Lenacapavir as PrEP among cisgender women in sub-Saharan Africa, showed similarly positive outcomes. These findings further strengthen the evidence base supporting regulatory approval of Lenacapavir as a Next-Gen PrEP Option.
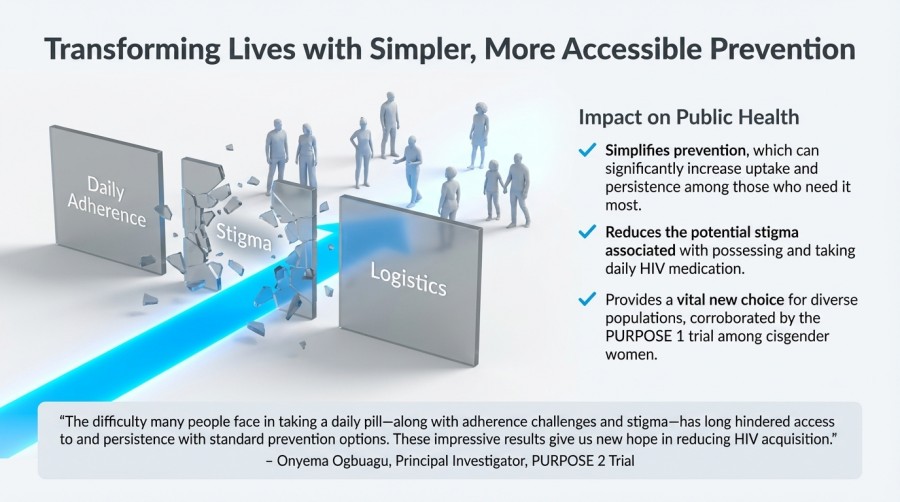
Public Health Impact of Lenacapavir
Lenacapavir has the potential to fundamentally reshape HIV prevention worldwide. If approved and widely accessible, its impact could include:
- A significant reduction in new HIV infections, particularly among high-risk populations
- Improved prevention effectiveness through twice-yearly dosing
- A shift toward more sustainable healthcare models with simplified prevention strategies
- Progress toward ending the HIV epidemic at the population level
- Expanded prevention choices beyond daily oral medication
- Continued advancement in HIV research and innovation
- A long-term goal of creating an HIV-free generation, supported by equitable access to high-quality healthcare
A Shared Responsibility for HIV Prevention
Everyone has a role to play in advancing HIV prevention—whether by sharing accurate information about PrEP or supporting efforts to make Lenacapavir widely accessible. Now is the time to work together toward a future free from AIDS, especially for communities that urgently need effective prevention tools. Education and awareness must remain at the heart of the global response, enabling us to build new hope and stronger opportunities for effective HIV prevention through Next-Gen PrEP Options.

References
Information related to the PURPOSE trials and the development of Lenacapavir is available through Gilead Sciences, along with additional research on HIV prevention and treatment advancements. Individuals seeking further guidance on HIV prevention or the use of Lenacapavir should consult qualified healthcare professionals for the most accurate and up-to-date information.

