In recent years, the concept of HIV treatment has advanced far beyond the use of ART (antiretroviral therapy) to suppress viral replication. Yet no technology has ever been able to truly eliminate latent reservoirs—the hidden HIV inside human cells—until now. A new study from China has developed a targeted HIV Gene-Editing system using engineered exosomes combined with CRISPR-Cas12a. This innovation represents a significant milestone that may reshape the future of HIV treatment.
Globally, many countries—including Thailand—are closely monitoring the rise of CRISPR-based HIV therapies. This article explains the scientific meaning behind this breakthrough, its connection to modern medicine, and how this next-generation HIV Gene-Editing system could impact healthcare in Thailand if it progresses to real-world clinical use.

HIV Gene-Editing – An Innovation Precisely Designed to Target the Virus
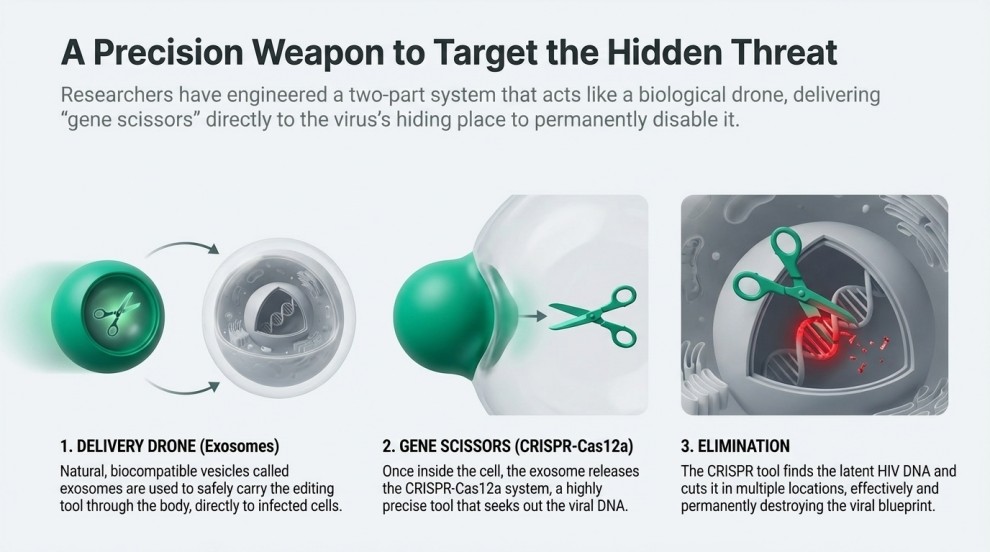
A research team from the University of Science and Technology of Wuhan, led by Gu Chaojiang, has developed a new delivery platform that uses exosomes to transport the CRISPR-Cas12a “gene scissors” into HIV-infected cells. Imagine a microscopic drone navigating deep into a hidden room, removing only its targeted object without damaging the structure around it—this is essentially how the new HIV Gene-Editing system works.
Key advantages of the new HIV Gene-Editing system
- Highly precise identification of viral DNA
- Enhanced safety—no need for high-dose viral vectors
- Ability to reach and disrupt latent HIV reservoirs
- Multi-site gene editing to prevent viral escape mutations
- Uses natural exosomes, making it more biocompatible
These features explain why this research has attracted significant global attention.
Why This HIV Gene-Editing Breakthrough Matters
Many may wonder why a new gene-editing method is such a big deal when gene-editing technology has existed for years. The answer lies in its ability to solve a challenge that the medical world has faced for over 30 years.
Major limitations of current HIV treatments
| Treatment Method | Advantages | Limitations |
|---|---|---|
| ART (Antiretroviral Therapy) | Suppresses viral load to undetectable levels; safe and widely used | Cannot eliminate latent HIV hidden in the nucleus |
| Immune Cell Therapy | Removes actively replicating cells | Cannot detect cells harboring latent HIV |
| Conventional Gene Therapy (AAV Vector) | Scientifically promising | Lacks targeting precision; risks toxicity |
To achieve a durable cure, we must eliminate latent HIV reservoirs—something no treatment has truly accomplished. The new HIV Gene-Editing system may be the closest solution ever achieved.
How the HIV Gene-Editing System Works
The HIV Gene-Editing system developed by Chinese researchers is designed to locate, target, and disrupt HIV DNA—both active and latent—inside infected cells. The process integrates advanced exosome engineering with CRISPR-Cas12a, creating a delivery mechanism capable of reaching viral reservoirs that traditional HIV treatments cannot eliminate. Below is a more detailed explanation of how each step works:

1. Engineering Exosomes
Action: Exosomes are biologically derived nanovesicles naturally released by cells. Researchers modify these exosomes to carry the Cas12a enzyme and program them with targeting molecules that recognize HIV-infected cells.
What Happens:
- Surface proteins are engineered so the exosomes can selectively bind to cell types commonly infected by HIV, such as CD4+ T cells.
- The exosomes encapsulate Cas12a safely, protecting it from degradation in the bloodstream.
- This ensures the gene-editing complex reaches the correct cells without causing widespread off-target exposure.
Outcome: Highly targeted delivery, allowing the therapy to reach even hidden viral reservoirs with minimal systemic toxicity.
2. Delivering Cas12a into the Cell
Action: Once the engineered exosomes reach the infected cell, they fuse with the cell membrane and release Cas12a and its guide RNA into the cytoplasm.
What Happens:
- Cas12a is transported into the nucleus—the location where HIV has integrated into the host’s genome.
- The guide RNA (gRNA) directs Cas12a toward specific sequences found in HIV proviral DNA.
Outcome: Cas12a is now positioned at the exact site where viral DNA needs to be edited, fully prepared for DNA cutting activity.
3. Locating HIV DNA (Active + Latent Reservoirs)
Action: Using the gRNA blueprint, the system scans the host DNA for HIV sequences.
What Happens:
- Cas12a can recognize both actively replicating HIV DNA and latent HIV DNA embedded silently in the genome.
- This ability to detect latent HIV is crucial because these reservoirs are untouchable by ART and responsible for viral rebound when treatment is stopped.
- CRISPR-Cas12a has higher precision and fewer off-target cuts compared to earlier Cas9 systems.
Outcome: Accurate localization of HIV DNA with minimal risk of off-target genome editing—one of the major safety advantages of this technology.
4. Cutting HIV DNA
Action: Once bound to the viral DNA, Cas12a performs a double-strand cut, breaking the HIV genome into fragments.
What Happens:
- Without an intact genome, HIV loses its ability to replicate or reactivate.
- Multi-site editing can be performed, meaning Cas12a can cut several regions of HIV DNA simultaneously to prevent viral escape mutations.
- The cell’s natural DNA repair mechanisms attempt to fix the break, but incorrectly repairing viral DNA leads to permanent inactivation.
Outcome: HIV enters a suppressed or non-functional state, significantly reducing or eliminating the viral reservoir inside the host cell.
5. Immune System Recovery
Action: With the viral genome disrupted, HIV can no longer hijack immune cells for replication.
What Happens:
- The immune system begins restoring CD4+ T-cell levels.
- Chronic inflammation associated with HIV infection decreases.
- The reduction in viral load offers the body a chance to rebuild immune function naturally.
Outcome: Significant reduction in viral load, improved immune markers, and potential progression toward a functional cure.
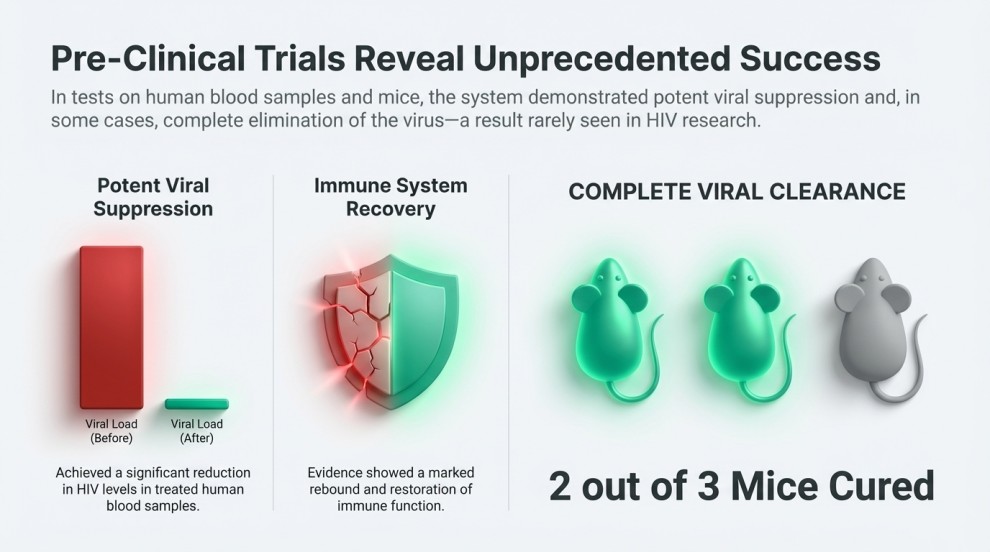
Evidence From Animal Studies
Animal trials demonstrated highly encouraging results:
- Several mice showed a strong reduction in proviral DNA after the HIV Gene-Editing treatment.
- In one study group, two out of three mice achieved complete viral clearance, meaning no detectable HIV DNA remained.
- Treated mice also exhibited improvements in immune cell counts and overall immune function.
These findings suggest that HIV Gene-Editing could become one of the first technologies capable of targeting—and potentially eliminating—latent HIV reservoirs, something no current HIV therapy can accomplish.
Promising Results from HIV Gene-Editing Experiments
Tests in mice and samples from people living with HIV showed:
- Strong reduction in viral DNA after HIV Gene-Editing
- Improved immune function
- In one cohort, two out of three mice achieved total viral elimination
Considering HIV’s ability to hide in human DNA for decades, achieving these results is an extraordinary scientific step forward.
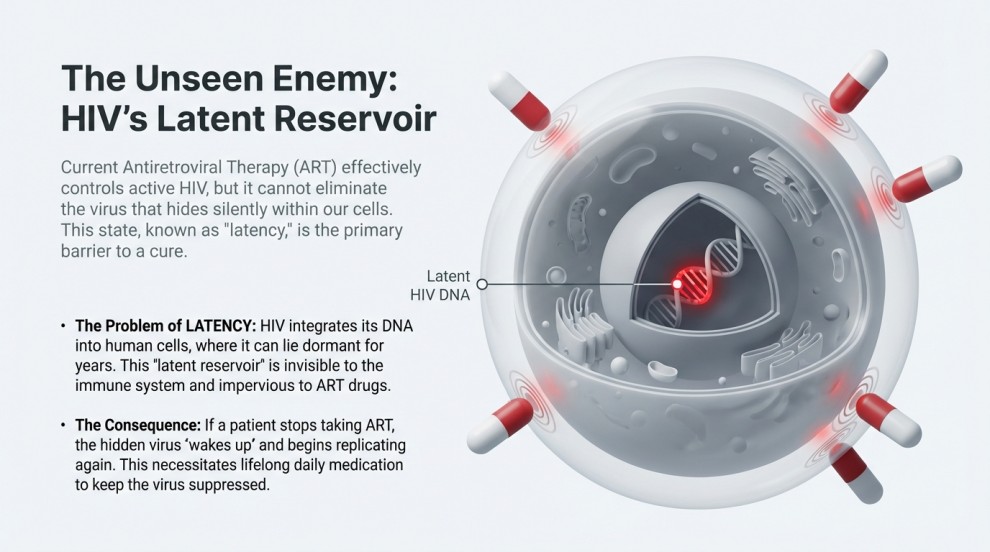
Why Eliminating Latent HIV Matters
Latent HIV enters a silent, non-replicating state known as latency. ART cannot target this form. If treatment stops, latent HIV reactivates instantly.
The new HIV Gene-Editing system may be able to:
- Identify hidden reservoirs
- Cut HIV DNA directly
- Push the virus into long-term suppression (functional cure)
This is something ART cannot achieve.
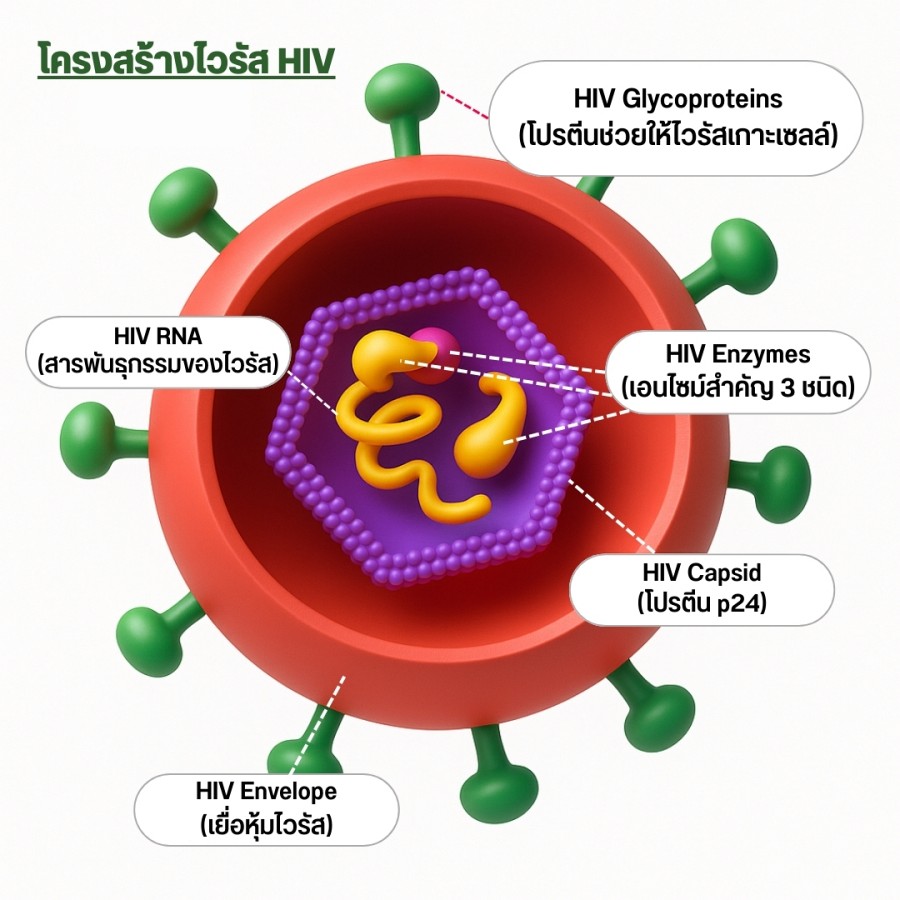
Understanding HIV Structure and Its Relevance to Gene Editing
- HIV Envelope
- A lipid membrane that protects the virus and helps it evade the immune system.
- HIV Glycoproteins (gp120 & gp41)
- Act as keys allowing HIV to bind CD4, CCR5, or CXCR4 receptors—initiating infection.
- HIV Capsid (p24)
- Protects viral RNA and enzymes; dissolves after entry into the cell.
- HIV RNA
- The genetic blueprint used to create new viruses; converted into DNA inside human cells.
- HIV Enzymes
- Reverse Transcriptase: Converts RNA → DNA
- Integrase: Inserts viral DNA into human DNA
- Protease: Builds new mature HIV particles
These components explain why HIV Gene-Editing must target the viral genome inside host DNA.
Overview: How HIV Works?
- HIV uses glycoproteins to attach to human cells → opening the entry pathway
- The capsid dissolves → releasing viral RNA and essential enzymes
- Reverse Transcriptase converts viral RNA into DNA
- Integrase inserts the viral DNA into the host cell’s DNA
- The human cell is then programmed to produce new HIV particles
- Protease processes viral proteins → assembling mature viruses that exit the cell and infect others
A Safer HIV Gene-Editing Method
Traditional gene therapies often require high doses, which increases toxicity. This new method uses exosomes, which are:
- Natural biological particles
- Less likely to trigger immune reactions
- Capable of specific targeting
- Small enough to penetrate tissues efficiently
These advantages make the new HIV Gene-Editing approach more suitable for human trials.
Next Step: Human Clinical Trials
The research team has confirmed that the project passed medical ethics review and is preparing for clinical trials. These trials will determine:
- Is the method safe for humans?
- Can HIV Gene-Editing work inside human tissues?
- Are there short- or long-term side effects?
- Is it effective across different stages of HIV infection?
If successful, this would mark a shift from simply suppressing HIV to genetically disrupting it.
What HIV Gene-Editing Could Mean for the Future
Potential Benefits
- A genuine possibility of functional cure
- Reduced reliance on lifelong ART
- Better immune restoration for advanced cases
- Adaptability for other viruses (e.g., HBV)
Challenges to Monitor
- Off-target editing risks
- Long-term safety
- Regulatory and ethical frameworks
- Real-world accessibility
Related Article
- Next-Level HIV Control – Research from Johns Hopkins
- Innovations in HIV Medication – A Path Towards a Cure
Broader Medical and Social Implications
Even though the new HIV Gene Therapy approach is still in early development, its success in initial experiments has sparked global optimism. If proven effective, society may witness:
- reduced lifetime medication burden
- improved quality of life for people living with HIV
- lower long-term healthcare costs
- major progress in destigmatizing HIV
ART already transformed HIV into a manageable condition.
But HIV Gene-Editing represents something far deeper
a technology that could disrupt HIV at the genetic root.

HIV Gene-Editing May Become a New Era of Treatment
This breakthrough may be the beginning of a realistic functional cure. The ability of HIV Gene Therapy to target and eliminate latent reservoirs marks a historic step in biomedical science. While human trials are still ahead, the potential is extraordinary. If future clinical studies validate its success, the world may witness a new chapter in HIV treatment—one that fundamentally changes how we understand and manage the virus.
References:
- นักวิจัยจีนพัฒนาระบบมุ่งเป้าตัดต่อยีน HIV ทำให้เชื้ออยู่ในภาวะสงบ
- จาก U=U สู่ CRISPR อนาคตการรักษา HIV ที่ต้องรู้ และสิ่งที่เราทำได้เลยวันนี้
- ดร.อนันต์เผยข่าวดีบริษัทในอังกฤษค้นพบวิธีรักษาผู้ป่วยติดเชื้อ
Last Updated on 11/12/2025 by ทีมที่ปรึกษา มูลนิธิเพื่อรัก