On June 18, 2025, the U.S. Food and Drug Administration (FDA) officially approved Yeztugo®—the brand name for lenacapavir, developed by Gilead Sciences—as the world’s first and only biannual injectable PrEP (Pre-Exposure Prophylaxis) for HIV prevention. This marks a major milestone in medical advancement that has drawn global attention. The approval is based on international clinical studies confirming that lenacapavir PrEP shot offers up to 99.9% effectiveness in preventing HIV infection, especially among high-risk groups such as men who have sex with men (MSM), LGBTQ+ individuals and serodiscordant couples (where one partner is HIV-positive and the other is not). Traditional PrEP requires daily oral medication, which, while highly effective, can be difficult for many to adhere to consistently—leading to increased risk of infection. But this year, the world is stepping into a new era with injectable PrEP, requiring just two injections per year to maintain protection.
A New Era in HIV Prevention: From Daily Pills to PrEP Shot
What is Lenacapavir PrEP Shot
Yeztugo® is the brand name for lenacapavir, a novel antiviral medication developed by Gilead Sciences. It works by targeting and inhibiting a key protein used by the HIV virus to replicate. This medication is the first capsid inhibitor in the world to be approved by the U.S. FDA for HIV prevention. Unlike traditional forms of PrEP, which usually inhibit enzymes like reverse transcriptase or integrase, lenacapavir directly targets the virus’s structural core, marking a significant innovation in antiretroviral therapy.

What is a capsid inhibitor?
A capsid inhibitor is an antiviral drug that interferes with the function of the capsid—the protein shell that encases the genetic material of the HIV virus. The capsid plays several essential roles in the HIV life cycle, including:
- Protecting the virus’s RNA
- Assisting the virus in entering human cells
- Facilitating the replication of the virus within the host
How does a capsid inhibitor work?
- It disrupts the process HIV uses to encapsulate its genetic material, weakening the virus.
- It prevents the release of viral RNA into target cells, blocking infection at the entry point.
- It interferes with the proper assembly of new viral particles, stopping the replication cycle.
- When the capsid can no longer function properly, HIV is unable to spread or reproduce efficiently in the body, making capsid inhibition a powerful new approach in the fight against HIV.
Key Benefits of PrEP Shot

1. Only Two Injections Per Year

PrEP Shot provides long-lasting protection for up to six months per dose, which means users only need two injections per year. This contrasts with daily oral PrEP, which requires consistent adherence to be effective. With fewer dosing intervals, injectable PrEP reduces the complexity of managing daily medication and provides long-term peace of mind, especially for those with unpredictable routines—such as frequent travelers, shift workers, or individuals who struggle to access medication regularly.
2. Reduces the Burden of Daily Medication
A major challenge with oral PrEP is the difficulty of maintaining a daily routine, including forgetfulness or fatigue from long-term pill use. While oral PrEP is highly effective, missing just a few doses can compromise its protective benefits. Lenacapavir, as an injectable PrEP, offers a practical solution for those unable to adhere to daily regimens or who have discontinued use in the past. With just two injections a year, it simplifies HIV prevention, reduces the psychological burden of daily medication, and may also minimize gastrointestinal side effects commonly associated with oral PrEP.
3. Greater Acceptance Among Key Populations
For some individuals, carrying or taking oral PrEP daily can feel inconvenient or expose them to unwanted scrutiny regarding their health or sexual behavior. This can lead to stigma or discrimination, especially in communities with limited understanding of HIV or PrEP. Injectable PrEP offers a more discreet and private alternative—no pills to carry, no visible signs of medication use. Receiving two injections per year in a clinical setting enables greater confidentiality, which in turn enhances acceptability and access among diverse at-risk populations.
4. Proven High Efficacy
Lenacapavir has been studied in large-scale clinical trials such as PURPOSE 1 and PURPOSE 2, conducted in countries including the United States, Kenya, and South Africa. These studies evaluated injectable PrEP in populations like men who have sex with men (MSM) and transgender women.
“The findings revealed that Lenacapavir reduces the risk of HIV infection by up to 99.9% when administered on schedule. No issues with drug resistance were reported, and most participants tolerated the medication well. Minor side effects, such as mild injection site pain, were short-lived and resolved on their own.”
Because of its outstanding efficacy and safety profile, many countries are now considering incorporating injectable PrEP like Lenacapavir into national healthcare programs as a crucial tool for ending new HIV infections in the future.
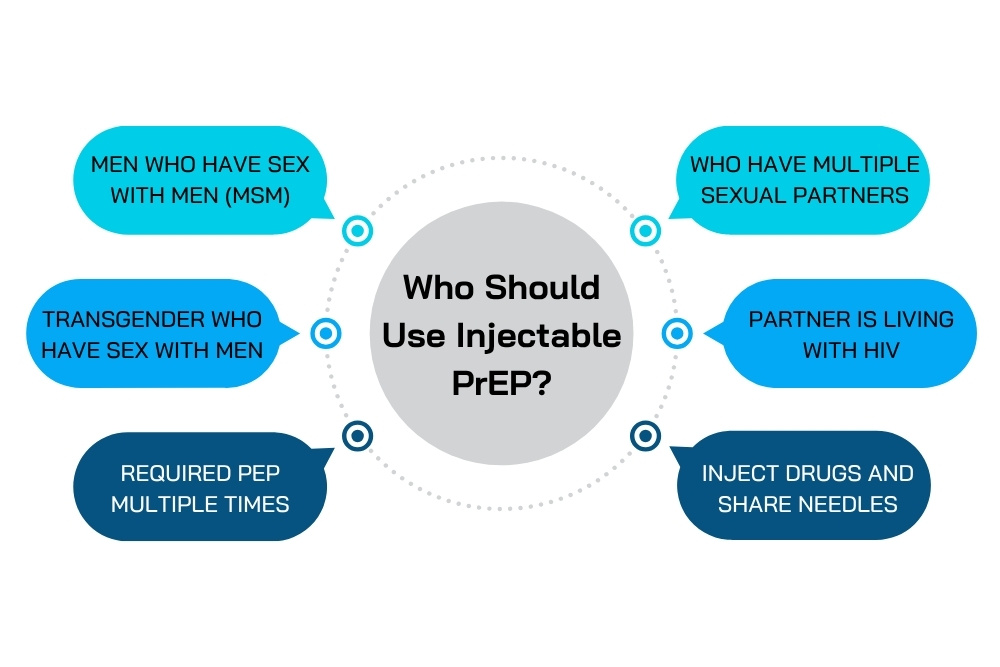
Who Should Use Injectable PrEP?
Injectable PrEP using lenacapavir is recommended for individuals at increased risk of HIV infection, including:
- Men who have sex with men (MSM) who do not consistently use condoms
- Individuals who have multiple sexual partners or frequently change partners
- Transgender individuals who have sex with men
- People in serodiscordant relationships (where one partner is living with HIV and viral suppression has not yet been achieved)
- People who inject drugs and share needles
- Individuals who have required PEP (Post-Exposure Prophylaxis) multiple times
The decision to start injectable PrEP should be made in consultation with a healthcare provider, taking into account the individual’s lifestyle, access to regular care, and ability to maintain consistent follow-up for injections every six months.
Steps to Start Injectable PrEP with Lenacapavir
Starting lenacapavir involves more than just the patient’s decision—it also requires comprehensive medical evaluation to ensure that the individual is physically and behaviorally ready. The process typically includes the following key steps:
1. Risk Assessment
The first step involves a detailed consultation with a healthcare provider to assess the individual’s risk of HIV infection. This may include:
- Recent sexual behaviors
- Use of injectable drugs
- History of PrEP or PEP use
- Whether the person has a partner who is HIV-positive or whose status is unknown
This step not only helps determine whether injectable PrEP is appropriate, but also opens up discussions about alternative options such as daily oral PrEP or consistent condom use.
2. HIV Screening
Before receiving any injection—especially the first dose—the patient must undergo an HIV test and receive a negative result to proceed. This is crucial because if a person is unknowingly HIV-positive and receives lenacapavir, it may contribute to antiviral resistance, as lenacapavir is designed for prevention, not treatment.
Acceptable HIV tests include Antigen/Antibody Tests, NAT (nucleic acid tests), or Rapid HIV tests, depending on the estimated time since possible exposure (i.e., window period).
3. Screening for Other STIs
In addition to HIV testing, individuals starting injectable PrEP should be screened for other common sexually transmitted infections (STIs), such as:
- Syphilis
- Gonorrhea and Chlamydia
- Herpes Simplex Virus (HSV)
- Hepatitis B and Hepatitis C (HBV and HCV)
These screenings not only benefit the individual’s health but also help prevent further community transmission, particularly in high-risk populations.
4. First Injection
Once all tests are complete and no medical contraindications are found (e.g., chronic liver disease, known allergy to any component of the drug, or other complications), the first lenacapavir injection is administered.
The medication is usually injected subcutaneously (under the skin) in areas like the abdomen or thigh. Unlike some medications that require intramuscular injection, lenacapavir’s method is generally less painful and more comfortable for most users. Most people report minimal discomfort and can resume normal activities immediately after the injection.
5. Ongoing Monitoring
Although injectable PrEP offers protection for about six months, routine follow-up is essential. Before each new injection, the patient must undergo an HIV test to confirm continued negative status. Additionally, physicians may monitor overall health, including liver and kidney function, and rescreen for STIs as part of a comprehensive sexual health plan. These follow-up visits help ensure continued safety, effectiveness, and early detection of any potential issues—not just related to HIV prevention but to overall sexual health.
Additional Information About Injectable PrEP
- The first injection is administered subcutaneously and may be combined with a short initial course of oral medication to help build up effective drug levels quickly.
- Subsequent injections are given every 26 weeks (approximately every 6 months).
- If a user misses a scheduled injection, doctors may prescribe oral PrEP temporarily until the next injection date.
- If another injection is missed again, repeat testing may be required, and the restart of injections will depend on clinical evaluation.
- Lenacapavir should not be used if the user is suspected to be HIV-positive or is showing symptoms of acute infection (such as fever, sore throat, or swollen lymph nodes).
- If the user wishes to stop using Lenacapavir, it is important to consult a healthcare provider to discuss alternative PrEP options or combined HIV prevention strategies.
Injectable vs. Oral PrEP: Which Is Right for You?
| Feature | Oral PrEP | Injectable PrEP |
|---|---|---|
| Frequency | Daily | Twice per year |
| Risk of Missing Dose | High | Minimal |
| Duration of Effect | Depends on adherence | Covers full 6 months |
| Side Effects | Some (e.g., nausea) | Mild, localized pain only |
| Privacy | Requires carrying pills | No daily reminders or medications |
Injectable PrEP in the Thai Context
Although some injectable PrEP options (like cabotegravir) are already available in Thailand, they require administration every 2 months. Currently, Lenacapavir has not yet been officially approved for use in Thailand. However, its emergence has prompted discussions about modernizing Thailand’s HIV prevention strategy. Thailand has several high-risk groups who could benefit significantly from long-acting injectable PrEP—such as:
- Gay and bisexual men in urban areas
- Sex workers
- LGBTQ+ youth who face social or familial barriers to accessing daily oral medication
Organizations working on HIV and sexual health are likely to play a crucial role in pushing for clinical trials or pilot programs to assess the use of Lenacapavir among Thai populations. Even today, despite access to knowledge and prevention tools, new HIV infections continue to occur, particularly among populations with limited access to care or persistent risky behaviors. The arrival of injectable PrEP like Lenacapavir is more than just a new medication—it represents a tangible source of hope. The more broadly PrEP access is expanded, the greater the potential to significantly reduce new HIV infections nationwide.
Frequently Asked Questions (FAQs) About PrEP Shot
☞ No. Injectable PrEP only protects against HIV, not other sexually transmitted infections like gonorrhea, syphilis, or hepatitis. Condom use remains essential for broader STI prevention.
☞ Yes. Lenacapavir does not interact with gender-affirming hormone therapy or contraceptives, and it can generally be used alongside many chronic medications. However, always consult your healthcare provider first.
☞ Yes. If medically assessed and deemed appropriate, Lenacapavir is suitable for both new and existing PrEP users, especially those seeking convenience and long-term protection.
☞ Users must receive an HIV test (result must be negative) and undergo screening for other STIs such as syphilis, gonorrhea, herpes, hepatitis B, and hepatitis C.
☞ Doctors may recommend using condoms during the first 7–14 days after the initial dose, until the drug reaches full effectiveness. Regular HIV testing is also advised before having unprotected sex.
Related Articles
Conclusion
The arrival of PrEP Shot specifically Lenacapavir (brand name: Yeztugo®), marks a major turning point in global HIV prevention. With just two injections per year, it offers highly effective protection against HIV, while reducing the burden of daily medication and increasing accessibility for individuals with time or lifestyle constraints. Its approval by the U.S. Food and Drug Administration (FDA) not only signifies scientific progress but also opens the door to new hope for reducing new HIV infections worldwide—particularly among high-risk populations who have been underserved by traditional PrEP methods. Although Lenacapavir has not yet been incorporated into Thailand’s national healthcare system, its promising clinical data and strong demand from key populations make it a likely candidate to become a key tool in the country’s future efforts to end HIV transmission.
Reference:
Yeztugo® (Lenacapavir) Is Now the First and Only FDA-Approved HIV Prevention Option Offering 6 Months of Protection
FDA Approves a Twice-Yearly Shot to Prevent HIV
- https://time.com/7295343/fda-hiv-shot-lenacapavir-yeztugo
US FDA approves Gilead’s twice-yearly injection for HIV prevention
- www.reuters.com/business/healthcare-pharmaceuticals/us-fda-approves-gileads-twice-yearly-injection-hiv-prevention-2025-06-18

